Abstract:
The EPO rejected two European patent applications, which both named an AI system as inventor, on the ground that the designation of inventorship did not fulfill the requirements of the EPC accepting only human persons as inventors. The EPO states in the decision that AI systems or machines cannot be holders of the rights conferred to an inventor by the EPC.
A closer look at the two patent specifications reveals that these do not contain any evidence that AI was plausibly involved in the inventive process of devising the claimed inventions.
The recent decision of the European Patent Office (EPO) to reject two patent applications for failure to name a natural person as inventor has attracted attention recently. Earlier this week the reasons for the decisions to refuse EP 3 564 144 and EP 3 563 896 were published on the EPO register website.
The identical applicant of both patent applications claims that the true inventor of the inventions disclosed in the applications is an Artificial Intelligence (AI) system called DABUS, a machine intelligence system developed by the applicant based on artificial neural networks allegedly being able to perform creative work. The applicant designated “DABUS” as the sole inventor of both patent applications and stated that he had obtained the right to the European patent from DABUS as a successor in title.
The EPO refused both applications under Art. 90(5) of the European Patent Convention (EPC) for formal reasons, because the requirements of Art. 81 EPC
“The European patent application shall designate the inventor. If the applicant is not the inventor or is not the sole inventor, the designation shall contain a statement indicating the origin of the right to the European patent.”
and Rule 19(1) EPC
“(1) The request for grant of a European patent shall contain the designation of the inventor. However, if the applicant is not the inventor or is not the sole inventor, the designation shall be filed in a separate document. The designation shall state the family name, given names and full address of the inventor, contain the statement referred to in Article 81 and bear the signature of the applicant or his representative.”
were not met. These requirements were interpreted by the EPO in such a way that the designated inventor has to be a human person. Mentioning the name of a machine thus does not fulfill the requirements.
In defense of his request to designate the AI system DABUS as the inventor, the applicant relied in particular on the following arguments:
- The Al system DABUS would be the actual deviser of the invention underlying the application. It would be a fundamental principle of patent law that the applicant must indicate the true inventor of the invention.
- Rule 19(1) EPC would not require that the inventor is a human person.
- Allowing an AI system or machine to be designated as inventor would be in line with the purpose of the patent system, namely to incentivise disclosure of information, commercialisation and development of inventions.
- Not accepting Al systems as inventors would exclude inventions made by Al from patentability, contrary to Articles 52-57 EPC.
The EPO based its decision to reject the application essentially on the following reasons:
- The legal framework of the EPC provides only for natural persons and legal persons to act within the system created by the EPC. Non-persons, i.e. neither natural nor legal persons do not have any role in proceedings before the EPO. In the context of inventorship reference is made only to natural persons. This indicates a clear legislative understanding that the inventor has to be a natural person.
- Names given to things may not be equated with names of natural persons. Names given to natural persons serve not only the function of identifying them but enable them to exercise their rights and form part of their personality. Things have no rights which a name would allow them to exercise.
- Under the EPC, the inventor has particular rights including the initial right to the European patent (which she can assign to a third party) and the right to be mentioned and designated as inventor on the publications of the application and the granted patent. Al systems or machines have at present no rights because they have no legal personality comparable to natural or legal persons. Legal personality is assigned to a natural person as a consequence of their being human, and to a legal person based on a legal fiction. Such legal fictions are either directly created by legislation, or developed through consistent jurisprudence. In the case of Al inventors, there is no legislation or jurisprudence establishing such legal fiction. It follows that Al systems or machines cannot have rights derived from being an inventor, such as the right to be designated as an inventor in the patent application. AI systems or machines also cannot transfer any rights such as the right to a European patent to a successor of title.
Consequently, the patent application was refused, because the designation of inventor filed by the applicant naming the machine “DABUS” as inventor does not meet the requirements of Art. 81 and Rule 19(1) EPC recited above. The decision is open to appeal by the applicant.
The EPO in accordance with Rule 19(2) EPC did not verify the claim that the AI system DABUS in fact is the inventor of the claimed invention. In the following we take a brief look at the two disclosures itself to check whether the AI system plausibly could be the inventor of the alleged inventions.
The first invention titled “Device and method for attracting enhanced attention” describes a device looking like a torch or pocket lamp having an LED light source 6 emitting light pulses having a fractal structure:
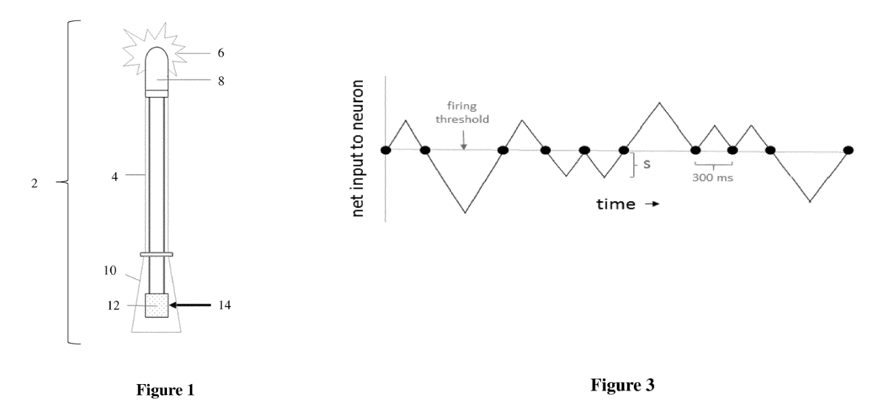
Claim 1 as amended after receipt of the search report from the EPO reads as follows:
- A device (2) for attracting enhanced attention, the device comprising:
(a) an input signal of a lacunar pulse train having characteristics of a pulse frequency of approximately four Hertz and a pulse-train fractal dimension of approximately one-half generated from a random walk over successive 300 millisecond intervals, each step being of equal magnitude and representative of a pulse train satisfying a fractal dimension equation of ln(number of intercepts of a neuron’s net input with a firing threshold)/ln(the total number of 300 ms intervals sampled); and
(b) at least one controllable light source (6) configured to be pulsatingly operated by said input signal;
wherein a neural flame is emitted from said at least one controllable light source as a result of said lacunar pulse train.
According to the description (paragraph [0017]), these particular light pulses defined in claim 1 should attract particular human attention:
“Even to the naked eye, and without the use of an anomaly detector, fractal dimension 1/2 pulse streams preferentially attract the attention of human test subjects. The most attention-grabbing aspect of such streams is that the ’holes’ or lacunarity between pulses occur as anomalies in what would otherwise be a linear stream of events. In other words, the pattern is frequently broken, such anomalous behavior possibly being detected by the TRN within the human brain as inconsistencies in the established arrival trend of visual stimuli. In contrast, should fractal dimension drop significantly below 1/2, the frequency of anomalous pulses drops, making them less noticeable to humans should either attention or gaze be wandering.“
The allegedly inventive light pulses having a fractal structure, however, are not generated by AI or machine learning but are simply received as an “input signal”. It is therefore not apparent at all, where the alleged machine intelligence system DABUS could have contributed to the invention. No evidence is submitted for the involvement of AI or neural networks or the like in conceiving the invention as defined by the above-recited claim 1. The claim features are not the result of a machine learning or other AI process. The allegation that DABUS is the real inventor seems not to be justified by the disclosure of the patent application.
The same applies to the second invention relating to a food or beverage container having a wall formed of fractal structures.
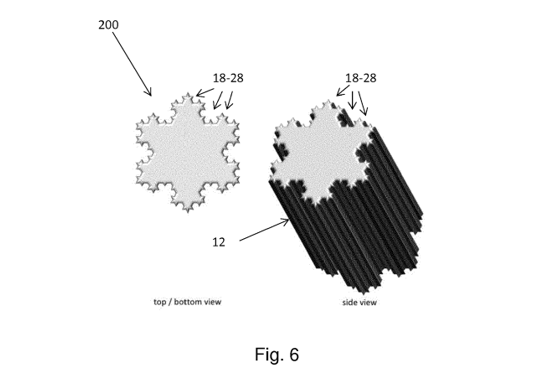
These fractal structures allegedly allowing coupling a plurality of such containers by inter-engagement, however, are likewise not described to be generated by AI. Again, a contribution of AI to the alleged invention is spurious.
The intention behind the two patent applications may actually be marketing-driven, namely to obtain a patent as an indication that the DABUS system indeed is able to perform creative tasks. The title “Device and method for attracting enhanced attention” then is aptly chosen.